Specific development
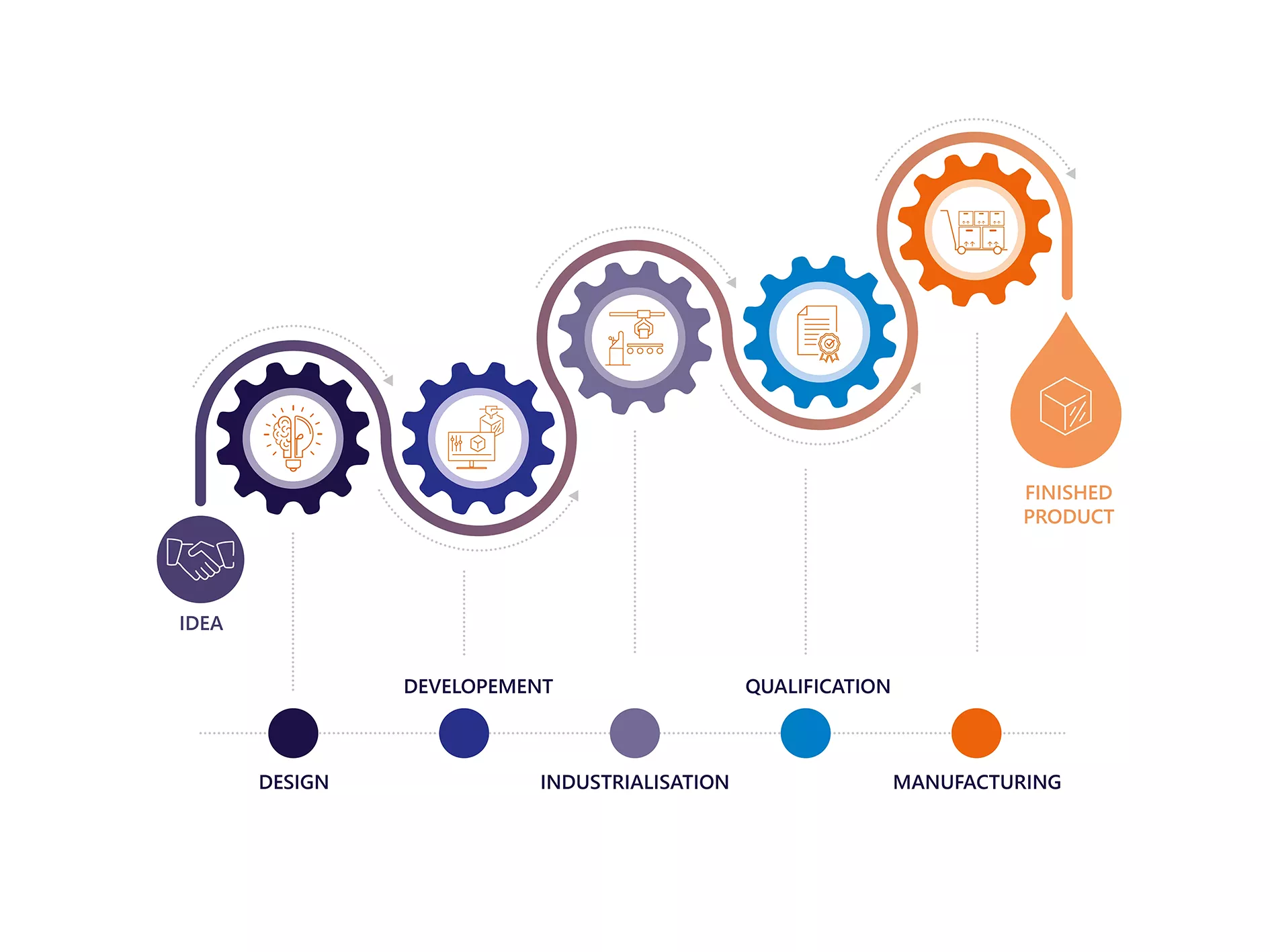
As a fully integrated group, SGH Medical Pharma designs and produces custom-made medical devices. We take charge of your idea from the design stage, based on your specifications and requirements, right through to delivery of the final product. With our injection know-how and strong industrial capabilities, we have the potential to become your contract manufacturer of choice to help you succeed.

Our development methods are governed by a strict and rigorous quality monitoring process, based on several stages:
A team dedicated to handling your concepts and projects
In the current global context of pressure on the supply of plastic raw materials, our specialist purchasing team ensures sustainable supplies as part of our business continuity plan.